Theme Leader :
Masashi Toyoda, Ph.D.
Researcher :
Norihiko Sasaki, Ph.D., Yoko Itakura, Ph.D., Kensuke Ohse, Ph.D.
Cardiovascular disease, Stem cell, Aging and senescence, Glycan
The dramatic expansion of the size of the elderly population increases risk of cardiovascular diseases, and creates additional burdens on medical economy. Therefore, the research on the effects of aging on cardiovascular diseases is a relevant topic. We aim at exploring the molecular mechanisms by which vascular homeostasis and cardiac regeneration are regulated during aging and disease process.
1. Vascular aging and diseases
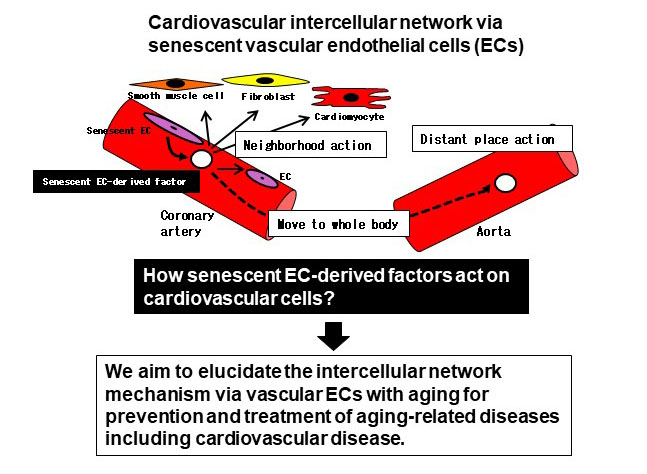
Vascular endothelial cells (ECs) play central roles in physiologically important functions of blood vessels and contribute to the maintenance of vascular homeostasis. We consider the impairment of EC functions as the development of vascular diseases and try to elucidate the molecular mechanisms, focusing on the glycoconjugates (e.g., glycoproteins, glycosphingolipids, and proteoglycans) mainly present on the cell surface.
In recent years, the involvement of senescent cell-derived factors in the onset and progression of cardiovascular disease has been pointed out, and importance of cell-cell interactions in the cardiovascular system is noted. However, it has not been clarified that what kinds of senescent EC-derived factor are working. We try to elucidate the intercellular network mechanisms of the cardiovascular system focusing on glycan-related secretory factors derived from senescent ECs.
2. Cardiac regeneration and aging
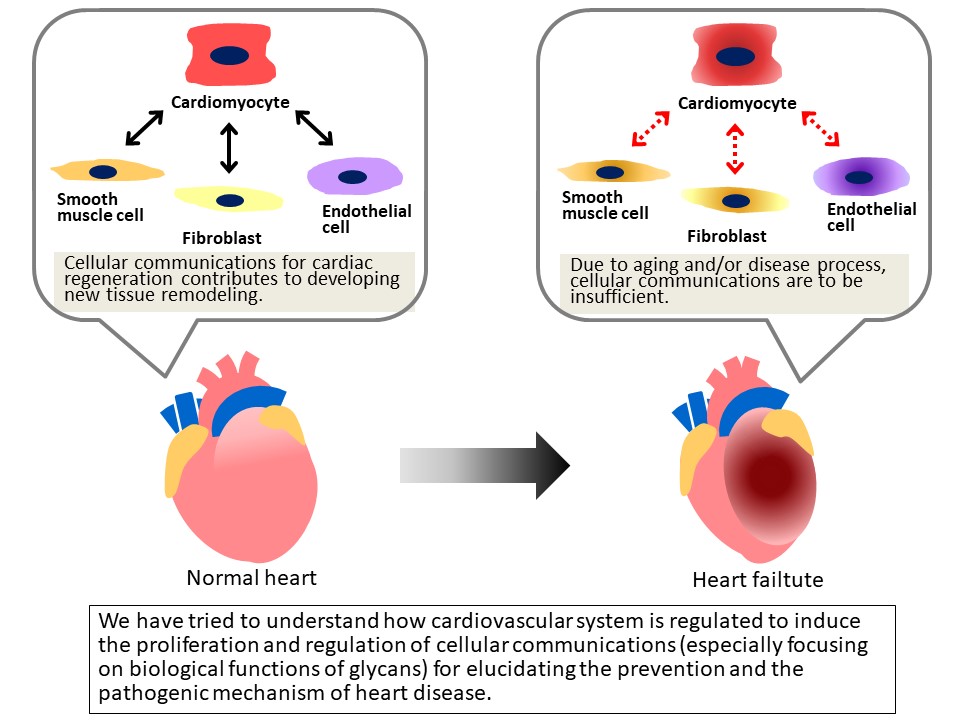
Understanding of cellular communications (e.g., cell-cell interaction, and cellular network) for cardiac regeneration contributes to developing new tissue remodeling strategy. To regenerate the damaged heart during aging and/or disease process, we aim at searching an alternative that enables us to increase the number of cardiomyocytes and to recover the heart functions. To this aim, we have tried to understand how cardiovascular system is regulated to induce the proliferation and regulation of cardiomyocytes, fibroblasts and endothelial cells, including their characteristic. Moreover, we have focused on cardiac stem cells, and have tried to clarify the role of the cells and realize the heart remodeling.
3. Stem cell biology for regenerative medicine
Stem cells have a capability to self-renew and differentiate into multiple types of cells and have varying degrees of differentiation potential: pluripotency, ES cells and iPS cells; multipotentiality somatic stem cells; and unipotentiality or precursor cells. Stem cell-based therapy has become a promising strategy for the treatment of many diseases. We are mainly focusing on stem cell biology and translational research for cardiovascular disease, which is one of the disease that will continue to grow in the future in the development of the aging society. In particular, we are promoting the validation to define and monitor the status of stem cells in vitro and in vivo using glycan profiling.
4. Development of the quality control method for regenerative medicine
Novel quality control method and criteria are essential for industrialization of regenerative medicine products. This is because regenerative medicine products contain live cells and exert the therapeutic effect through multiple mode of action. We are developing the appropriate evaluation method of regenerative medicine products which are different from conventional medicine.
Five Long-term Longitudinal Studies: Tokyo-LSA
Healthy Aging Innovation Center
Integrated Research Initiative for Living Well with Dementia